Introduction
‘Forensic Medicine’ or ‘Legal Medicine’ is the application of knowledge and principles of medicine for the purpose of law, both civil and criminal. Its main objective is to aid in the administration of justice.1
Forensic experts summoned to the scene of death are aimed to provide an estimation of the time elapsed since death. In forensic medicine and thereby criminal law, the important problem is to estimate the time of death. In the court of law, a medical practitioner has to give evidence as a medical jurist in order to prove the innocence or guilt of an accused. He has a great responsibility that he may be the only reliable evidence in the court of law.2
Post mortem interval is the interval between the death and time of examination of a body. This is important in knowing when the crime was committed. It helps police to start their inquiries with the available information and also in dealing the cases more efficiently. It also helps in including and excluding the suspects and culprits and in confirming the statements of the suspect. Estimation of time since death is useful in civil cases such as inheritance of property, insurance claims etc.3
Longer the post mortem interval, wider will be the limits of probability. Though the changes like cooling of body, eye changes, post-mortem staining, rigor mortis, stomach contents, bladder and bowel contents, decomposition changes and circumstantial evidence can sometimes yield a reasonable accurate result in early post-mortem hours, they are not reliable because of environmental factors. Hence forensic pathologists and biochemists have been concentrating on biochemical changes that occur in body fluids such as blood and compartmental fluids like vitreous humour, cerebrospinal fluid, pleural fluid, pericardial fluid and synovial fluid.4
Because of the lack of oxygen in the circulation, alteration in the enzymatic reaction and stoppage of production of metabolites, extensive biochemical changes occur in all body fluids. Such changes provide chemical markers which help to determine time of death accurately. 5
These metabolites are products of metabolism and intermediates of smaller molecular size which have functions in normal growth and development of cells. They may be exogenous or endogenous changes and hence metabolite profiles are altered following death.6
The changes that occur after death have been identified and attributed to agonal period, changes in the early post-mortem period and diffusion of substances between various body fluids.
Biochemical markers are divided into two classes - metabolites and proteins. Sodium, potassium, chloride, calcium, magnesium, phosphate, lactic acid, hypoxanthine, urea, creatinine, uric acid, ammonia, catecholamines, ethanol are metabolites. Other class includes total proteins and enzymes aspartate aminotransferase and lactate dehydrogenase.
Immediately after death, the changes that occur in the chemical composition of body fluids such as blood, vitreous humour, synovial fluid and cerebrospinal fluid are described by thanatochemistry. After death, the electrolytes and the chemicals redistribute, cellular integrity is lost, energy dependent trans membrane transportation is absent. Hence it will be difficult to assess post-mortem blood samples. Because of the breakdown of the active membrane transport and rapid breakdown of metabolism after death, only stable analytes can be estimated in blood samples.7
Among the various body fluids like blood, serum, cerebrospinal fluid, aqueous humour, synovial fluid and vitreous humour, estimation of concentration of potassium in vitreous humour is the most widely used method.8
Vitreous fluid is an acellular , transparent, inert, colourless , hydrophilic viscous fluid that is present between the lens and retina within the eyeball which is an important supporting structure that serves the optical function. Its weight is approximately 4 grams and its volume is approximately 4 cc. It is composed of 99% of water with soluble proteins, amino acids, low molecular weight constituents, glucose, type II collagen, hyaluronic acid, inorganic salts and ascorbic acid.9, 10
It is more than 40 years that the biochemical changes in vitreous have been on analysis. The underlying principle in analysing vitreous humour is that it is a closed compartment which is separated from the rest of the body. But ambient temperature may influence.
The composition of the vitreous is closely related to that of serum, aqueous humour and cerebrospinal fluid. It is relatively stable, easily accessible, less susceptible to rapid chemical changes, well protected from decomposition and contamination. Hence it is more suitable for analysis than other fluids in estimating time since death.11
Eye and thereby Vitreous humour is well protected even in case of severe head injury and in burns. This was frequently remarked as “miraculous Escape”.12
Many studies have been conducted on analysis of sodium and potassium in vitreous humour.13, 14, 15
Concentration of sodium, potassium and urate were analysed in vitreous humour of both the eyes at the same time of death showed variation between the eyes.16
Many studies have shown that the vitreous potassium level increases with increase in post-mortem interval. 4, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41
There was a linear relationship of increase in potassium concentration with increase in postmortem interval.42
As the post mortem interval increases, the potassium also increases whereas there is negative correlation with sodium and no correlation with chloride and calcium.43
Sodium concentration in normal body is 136-145 mEq/L. Potassium concentration in normal body is 50-55 mEq/kg of body weight of which 160 mmol/l ie. 98% of the potassium exists within cell whereas extracellular concentration is 3.5-5.5 mmol/l.44 The normal sodium value in vitreous humour is 118-124 mmol/L and potassium value in vitreous humour is 2.6-4.2 mmol/L.4 Potassium is actively transported from ciliary body into anterior vitreous and posterior chamber. Potassium is also contributed by the lens.44
Materials and Methods
This study was carried out on 100 cases which were brought for medico-legal autopsy at Rajiv Gandhi Government General Hospital, Madras Medical College, Chennai-3. Cases which were admitted in hospital and whose exact times of death were known were selected for the study. Details of these cases were obtained from the hospital records, police records, relatives and friends. Cases whose exact time of death was not known and with previous history of eye or orbital injury or surgery, posterior segment diseases were excluded from the study.
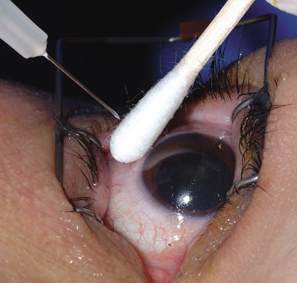
Vitreous humour was collected from both eyes. First sample of vitreous humour was aspirated from both the eyes simultaneously as early as possible after the entry of the body into mortuary. Second sample was taken from both the eyes simultaneously at the time of post-mortem examination. The samples which were turbid and mixed with blood were discarded. The details of the cases were recorded.
Samples of vitreous humour were collected from the posterior chamber by aspirating gradually and slowly through a puncture 5-6 mm away from the limbus using a sterile 20 gauge needle taking care to avoid tearing of any loose tissue fragments surrounding the vitreous chamber.
The samples were sent to the Institute of Biochemistry, Rajiv Gandhi Government General Hospital, Madras Medical College, Chennai-3.
The samples were analysed by Medica Easylyte Sodium/potassium analyser. It is a fully automated, electrolyte system that is microprocessor controlled. It uses Ion Selective Electrode(ISE) technology for the measurement of electrolytes in which are stored, an easily accessible quality control data. It measures sodium, potassium, chloride, lithium, calcium and pH of the serum, whole blood, plasma, urine, vitreous and synovial fluid. The calibrants are packed in a convenient solution pack with
Standard A solution, 800 ml (140.0 mmol/L sodium *4.0 mmol/L potassium *Buffer* Preservative *wetting agent).,
Standard B solution 180 ml (35.0 mmol/L sodium,16.0 mmol/L potassium *Buffer* Preservative*Wetting agent)
Wash solution, 80 ml (0.1 mol/L (Ammonium bi fluoride)
Ion selective Electrode method consists of a thin membrane across which only the intended ion can be transported. The transport of ions from a high concentration to a low concentration through a selective binding with some sites within the membrane creates a potential difference which is measured in Volts.
Observations
This study was carried out on 100 cases whose time of death was known who were brought to the mortuary, Rajiv Gandhi Government General Hospital, Madras Medical College, Chennai-3 for post-mortem examination.
Samples of vitreous humour were taken at intervals. First sample was taken as early as possible after the entry of the dead bodies into the mortuary from both the eyes. Second sample was taken at the time of post-mortem examination from both the eyes.
Only the clear vitreous humour was analysed. Turbid or blood stained samples were excluded from the study.
The samples were analysed for sodium and potassium by selective ion electrode method.
Table 1
Percentage of cases depending upon Time since death
This Table 1 shows percentage of cases depending upon time since death in sample 1 and sample 2 of vitreous humour of both eyes.
Table 2
Descriptive for Sample I & II – Time since death
Table 3
Regression co-efficients for Vitreous Fluid
Table 4
Correlation between TSD and K and Na Values
Table 5
Group statistics
Table 6
T-Tests
Table 7
Oneway ANOVA for Comparing Left and Right Na and K values for Three periods (0 to12, 12 to 24 and Above 24 hrs Sample 1 & II
Table 1 shows number and percentage of cases from sample 1 and 2 which falls within the time intervals. It was concluded that in sample 1, there were 53 cases with time since death within 12 hours which constituted 53%, there were 40 cases with time since death from 12.1 to 24 hours which constituted 40% and 7 cases with time since death more than 24 hours which constituted 7% out of 100 total cases. In sample 2, there were 31 cases with time since death within 12 hours which constituted 31%, there were 57 cases with time since death from 12.1 to 24 hours which constituted 57% and 12 cases with time since death more than 24 hours which constituted 12% out of 100 total cases.
Table 2 shows total number of cases N, Mean, Standard deviation, standard error, 95% confidence interval for mean and the range of sodium and potassium values of sample I & II of right and left eye. Each group have different values (<12 hours, 12.1-24 hours and >24 hours). The table depicts p< 0.001 which is a statistically significant data which concludes the positive correlation of time since death with potassium increase whereas sodium level falls as time increases. Hence it is not statistically significant.
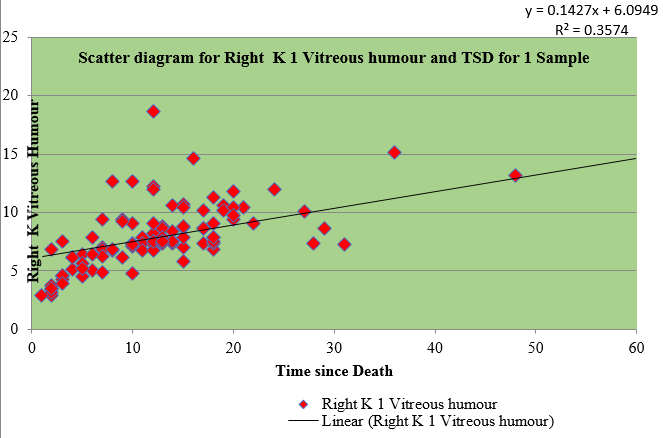
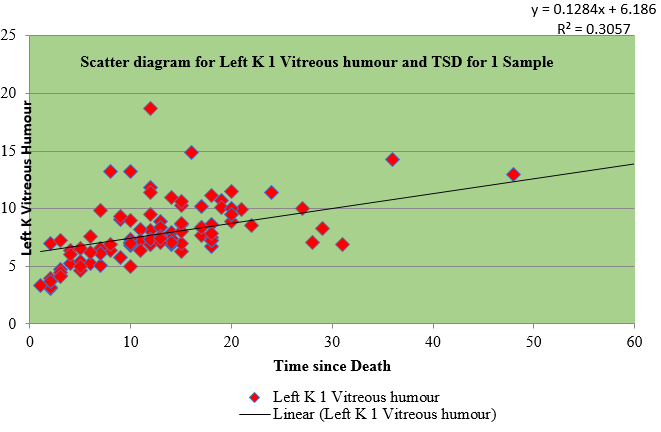
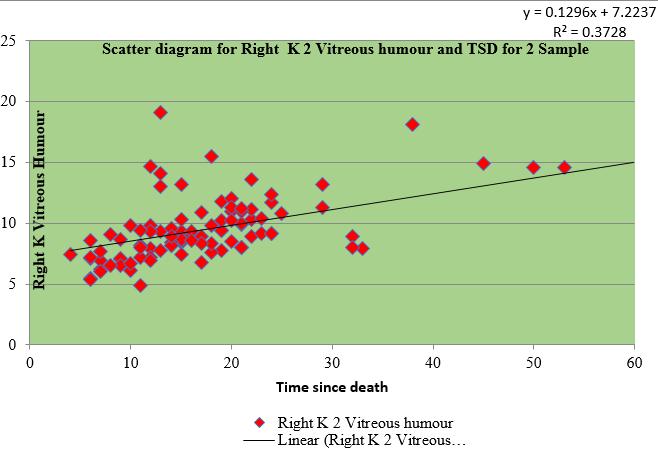
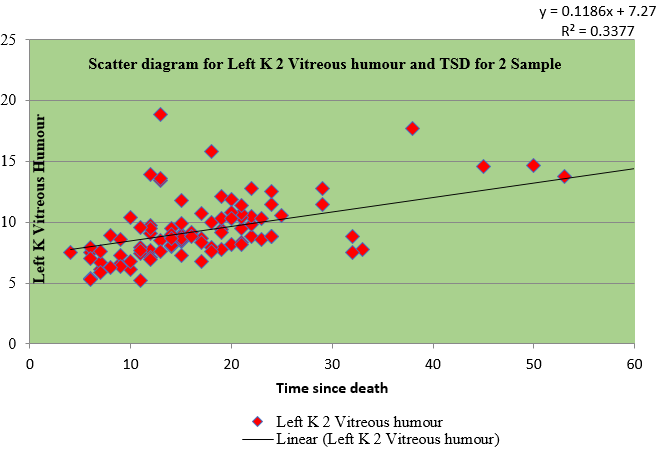
Table 3 shows the regression for sample I and II of right and left eye vitreous fluid with time since death as a dependent variable. The values of vitreous potassium and time since death were significantly correlated(r=0.450), (r=0.421), (r=0.574), (r=0.567)
Table 4 shows the Pearson coefficient of 0.580 of sample I of right vitreous potassium, 0.536 of sample I of left vitreous potassium, 0.611 of sample II of right vitreous potassium and 0.581 of sample II of left vitreous potassium which has a significant correlation with time since death whereas sodium has negative correlation.
Table 5 shows the comparison of sodium and potassium values of sample 1 and 2 of right and left vitreous. Statistical analysis shows P>0.05 which concludes there is no significant difference between right and left values.
Table 6 shows the T test which compares the values of sodium and potassium of sample 1 and 2 of right and left vitreous with timing 0-12 hours,12-24 hours, and more than 24 hours. Statistical analysis shows p>0.05 for sodium values which is not significant whereas p<0.05 for potassium is significant. which concludes there is no significant difference.
Table 7 shows the one way ANOVA for comparing sodium and potassium values of both sides for three periods in sample I and sample 2 respectively. It shows p<0.001 which is a statistically significant data which concludes that there is a linear correlation of increase in potassium with time since death.